

What is the molecular shape of #"PCl"_5#?Ītoms past #"Si"# in the Periodic Table can “expand their octet” and have more than eight valence electrons. In water, the observed #"H-O-H"# bond angle is 104.5°.Īll bond angles in #"AX"_2"E"_2# molecules are significantly less than 109.5°. The #"H-O-H"# bond angle is less than that in #"NH"_3#, partly because of the greater repulsions caused by two lone pairs. The electron pair geometry of #"H"_2"O"# is tetrahedral. The central atom, #"O"#, has four groups bonded to it, two hydrogen atoms and two lone pairs. What is the molecular geometry of #"H"_2"O"#? #"AX"_2"E"_2# - two bond pairs and two lone pairs The greater repulsion of the lone pair causes the #"H"# atoms in #"NH"_3# to be bent closer together than the normal tetrahedral angle of 109.5°. The #"NH"_3# pyramid has a triangular base. If we look only at the atoms, we see a short, rather distorted tetrahedron. Remember that, in determining the molecular shape, we consider only the positions of the atoms, not the lone pairs. The electron pair geometry of #"NH"_3# is tetrahedral. The central atom, #"N"#, has four groups bonded to it: three hydrogen atoms and a lone pair. What is the molecular geometry of #"NH"_3#? #"AX"_3"E"# - three bond pairs and one lone pair. The shape of the #"CH"_4# molecule is tetrahedral. The 109.5° angle is the same for all #"H-C-H"# bond angles and is called the tetrahedral bond angle.

This shape minimizes the repulsion between the bond pairs. The four bond pairs are arranged about the #"C"# atom, pointing toward the corners of a regular tetrahedron. The shape of this molecule, however, is not planar, as you might think from the way we draw this dot structure. They compress the bond angle between the oxygens and sulfur to about 119.5°. The lone pair of electrons occupies a relatively large volume, since they are held by only one atom. Hence, the molecular shape of #"SO"_2# is bent and is represented as In determining the molecular shape, we consider only the positions of the atoms, not the lone pairs. The molecular shape of #"SO"_2# is not trigonal planar. The electron pair geometry of #"SO"_2# is trigonal planar. The central atom, #"S"#, has three groups bonded to it, two oxygen atoms and a lone pair. #"AX"_2"E"# - two bond pairs and one lone pair The shape of the molecule is trigonal planar.Īll the atoms are in the same plane, and the #"F-B-F"# bond angles are all 120°. Minimizing the repulsion causes the #"F"# atoms to form an equilateral triangle about the #"B"# atom, as shown below. The #"B"# atom has three bond pairs in its outer shell. The shape of the molecule is linear, and the #"Cl-Be-Cl"# bond angle is 180°. Repulsion between these two pairs causes the atoms to be as far apart as possible. The central #"Be"# atom has two bond pairs in its outer shell (SN = 2). The Lewis dot structure for #"BeCl"_2# is Lone pairs repel more than bond bonding pairs.
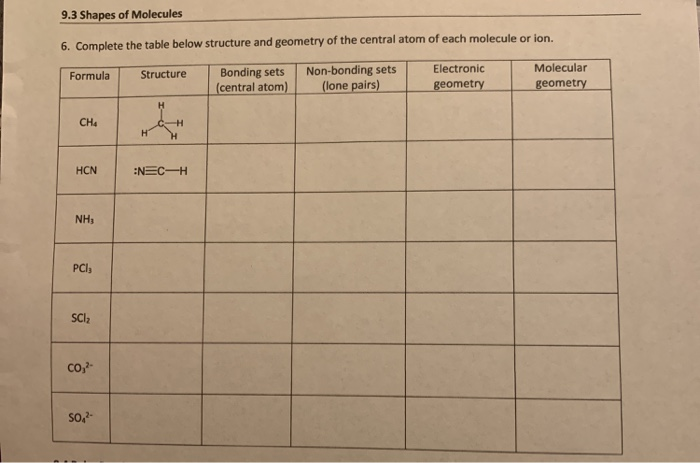
You must determine the steric number (SN) - the number of bonding pairs and lone pairs about the central atom. The repulsion between valence electron pairs in the outer shell of the central atom determines the shape of the molecule. Use the VSEPR shape to determine the angles between the bonding pairs. Use the SN and VSEPR theory to determine the electron pair geometry of the molecule. That gives you the steric number (SN) - the number of bond pairs and lone pairs around the central atom. Write the Lewis dot structure of the molecule. There are three basic steps to determining the molecular shape of a molecule:


 0 kommentar(er)
0 kommentar(er)
